Guillain-Barré Syndrome Linked to the RSV Vaccine

The potential risk of Guillain-Barré syndrome (GBS) developing after receipt of Pfizer’s respiratory experimental syncytial virus (RSV) vaccine have prompted the U.S. Food and Drug Administration (FDA) to request that Pfizer conduct a post-marketing safety study if the vaccine is approved for use in the United States this spring.1 The RSV vaccine manufactured by GlaxoSmithKline […]
Taiwan Compensates Family of Girl Who Died Days After Getting Second Dose of Pfizer’s COVID Shot
The Ministry of Health and Welfare of Taiwan has agreed to pay $114,829 under the National Vaccine Injury Compensation Program (VICP) to the family of a young girl in Taoyuan, Taiwan, who died of myocarditis (inflammation of the heart muscle) within days of receiving the second dose of Pfizer/BioNTech’s Comirnaty COVID-19 messenger RNA (mRNA) shot. […]
Shingles May Be Triggered by Covid Shots
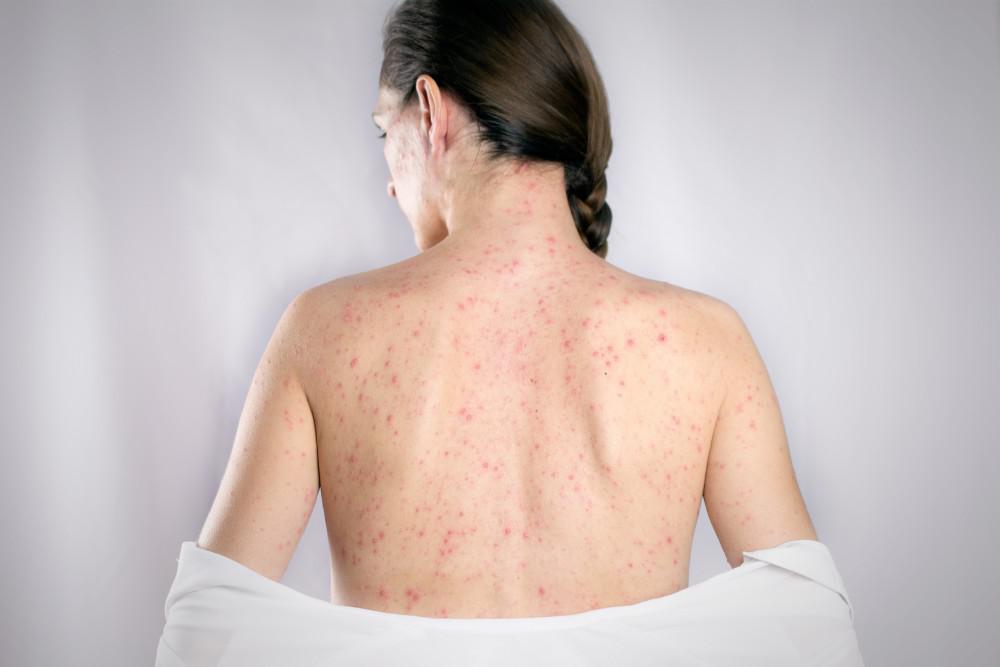
A large retrospective cohort study conducted by researchers in Germany and the University of Virginia compared the incidence of shingles among individuals who did and did not get a Covid-19 shot and found a statistically significant difference providing evidence for an association between Covid vaccinations and increased risk for developing shingles or herpes zoster (HZ). […]
South Africa Confirms Second Guillain-Barré Syndrome Death Due to COVID Vaccine
The South African Health Products Regulatory Authority (SAHPRA) confirmed on Sept. 12, 2022 that another person in South Africa has died of a polio-like neurological disorder that causes paralysis known as Guillain-Barré syndrome (GBS) due to receiving Johnson & Johnson/Janssen’s adenovirus vectored Ad26.COV2.S COVID-19 vaccine. SAHPRA said the National Immunization Safety Expert Committee (NISEC) performed […]
Death in South Africa Causally Linked to Johnson & Johnson COVID Vaccine
On Aug. 4, 2022, the South African Health Products Regulatory Authority (SAHPRA) reported the death of an individual in South Africa shortly after receiving Johnson & Johnson/Janssen’s adenovirus vectored Ad26.COV2.S COVID-19 vaccine. The agency stated that the death was causally linked to the vaccine.1 2 3 4 5 The name and age of the person […]
FDA Grants EUA for Novavax’s COVID Vaccine
Opinion | On July 13, 2022, the U.S. Food and Drug Administration (FDA) granted Emergency Use Authorization (EUA) to Novavax to distribute its experimental two-dose NVX-CoV2373 (also known as “Nuvaxovid” and “Covovax”) COVID-19 vaccine for use by adults. The decision was expected, given the favorable recommendation by the FDA’s Vaccines and Related Biological Products Advisory […]

