FDA’s Expert Panel Recommends COVID-19 Booster Dose for Seniors and Others, Rejects Booster Dose for Children

Despite not convening the Vaccines and Related Biologic Products Advisory Committee (VRBPAC) last month to vote on effectiveness and safety of the Pfizer-BioNTech COVID-19 vaccine (licensed under the name COMIRNATY), the U.S. Food and Drug Administration (FDA) convened the advisory committee on Friday, Sept. 17, 2021 to vote on booster doses of the vaccine.1 The […]
Vaccinated Americans Count Their Blessings Despite Getting COVID

On Aug. 2, 2021, Senator Lindsey Graham of South Carolina became the first fully vaccinated (for COVID-19) U.S. Senator to disclose he had tested positive for the SARS-CoV-2 virus. Sen. Graham said he had begun to experience “flu-like symptoms” two days earlier and eventually decided go to the doctor and get tested.1 Despite becoming another […]
Robert Malone, MD on the Availability of Comirnaty

[Comirnaty] is absolutely not available. So the little trick that they have done here, is [the U.S. Food and Drug Administration] issued two separate letters for two separate vaccines. The Pfizer vaccine, which is what currently available is still under Emergency Use Authorization… The product that’s licensed [by the FDA] is the BioNTech product, which […]
Guillain-Barré Syndrome Warning Added to AstraZeneca’s Vaxzevria COVID Vaccine in Europe

The European Medicines Agency (EMA) last week added an autoimmune condition known as Guillain-Barré syndrome (GBS) as a possible side effect to the product information on AstraZeneca/Oxford University’s Vaxzevria (or “AZD1222”) experimental adenovirus vectored vaccine. GBS is a neurological disorder that causes pain, numbness, muscle weakness and sometimes paralysis.1 2 3 4 A total of […]
The FDA Has a History of Rushed Drug Approvals

Two weeks ago, the Pfizer COVID-19 vaccine was reported as the first COVID-19 vaccine to receive full approval from the U.S. Food and Drug Administration (FDA) for individuals aged 16 and older.1 But, for some, questions remain regarding conflicting discrepancies of the wording surrounding the approval.2 While the FDA’s report states that full approval was […]
FDA Approves BioNTech’s Comirnaty. Pfizer COVID Shot Remains Experimental.
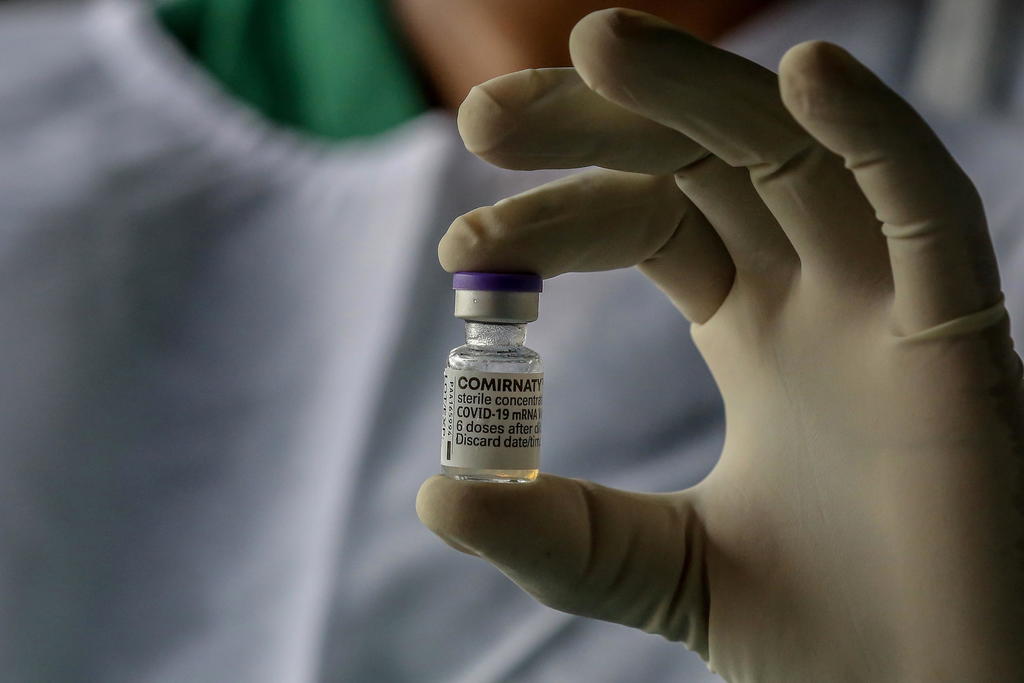
On Aug. 23, 2021, the U.S. Food and Drug Administration (FDA) gave full approval to a Biologics License Application (BLA) submitted by BioNTech Manufacturing GmbH of Mainz, Germany on May 18, 2021 for a biologic drug called COVID Vaccine, mRNA. The FDA gave permission to BioNTech to label the product “Comirnaty” and market it in […]

