Study: Natural Immunity Offers More Protection Than Three mRNA COVID Shots

As the debate continues surrounding the issue of bodily autonomy being violated by school and workplace COVID-19 vaccine mandates, a new research study that analyzed more than one million people has found that natural immunity to SARS-CoV-2 offers longer lasting protection than vaccination.1 The study, which was published in The New England Journal of Medicine […]
German Report Reveals 1 in 25 Insured Individuals Treated for COVID Shot Reactions
Techniker Krankenkasse, Germany’s largest health insurance company, reported that 437,593 of 11 million (1 in 25) insured individuals in 2021 had to undergo medical treatment as a result of adverse reactions from COVID-19 shots.1 COVID shots currently approved for use in Germany include Novavax’s Nuvaxovid, Moderna’s Spikevax, Pfizer/BioNTech’s Comirnaty, Johnson & Johnson/Janssen’s Ad26.COV2.S, AstraZeneca/Oxford University’s […]
Herpes Zoster Reactivated Following mRNA COVID Shots, Studies Show
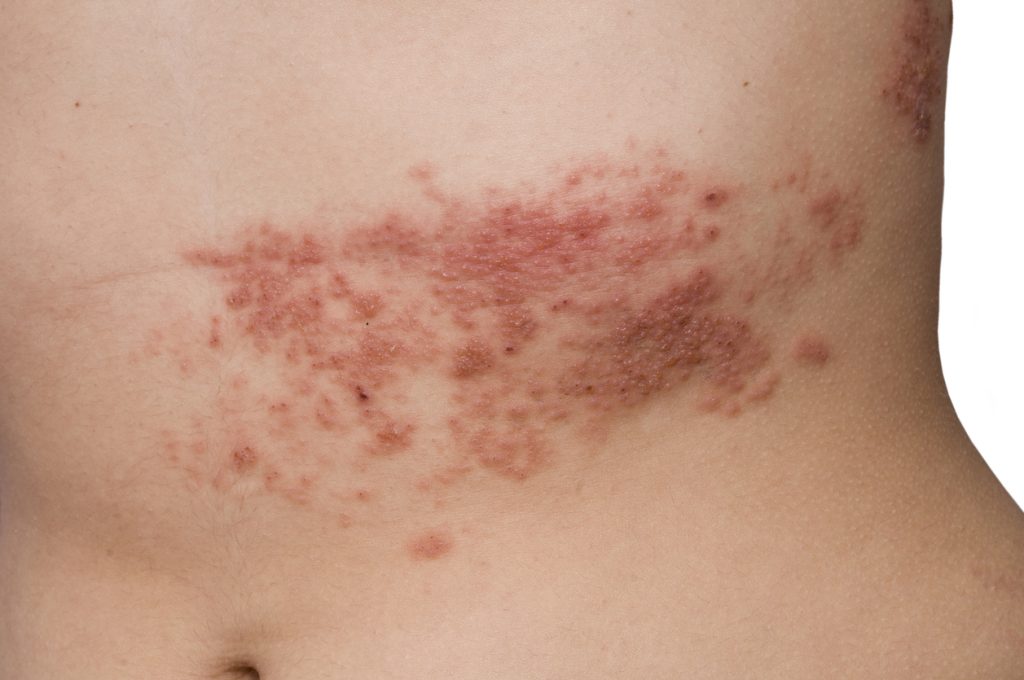
Concerns about the adverse effects of mRNA COVID-19 shots continue to grow a year and a half after they began to be distributed under an Emergency Use Authorization (EUA) granted to Pfizer/BioNTech and Moderna by the U.S. Food and Drug Administration (FDA). Cases of herpes zoster (HZ) following messenger RNA (mRNA) COVID shots have been […]
Japanese Government Pays Family for Death Tied to COVID Shot

On July 25, 2022, a committee of Japan’s Ministry of Health, Labor and Welfare announced that it would provide a lump-sum death benefit of ¥44.2 million ($324,000), plus an additional ¥212,000 for funeral expenses, to the family of an elderly woman who died shortly after receiving a COVID-19 shot. The committee determined there was a […]
FDA Grants EUA for Novavax’s COVID Vaccine
Opinion | On July 13, 2022, the U.S. Food and Drug Administration (FDA) granted Emergency Use Authorization (EUA) to Novavax to distribute its experimental two-dose NVX-CoV2373 (also known as “Nuvaxovid” and “Covovax”) COVID-19 vaccine for use by adults. The decision was expected, given the favorable recommendation by the FDA’s Vaccines and Related Biological Products Advisory […]
Study Shows Sperm Concentration Decreased Three Months Following Pfizer’s COVID-19 Vaccinations
In a new study published in the journal Andrology, researchers found what they believe to be a temporary decline of sperm concentration and total motile count among sperm donors in Israel three months following vaccination with Pfizer/BioNTech’s BNT162b2 (also known as “Comirnaty”) messenger RNA (mRNA) COVID-19 biologic.1 Study Investigates the Effect of the Pfizer COVID […]

